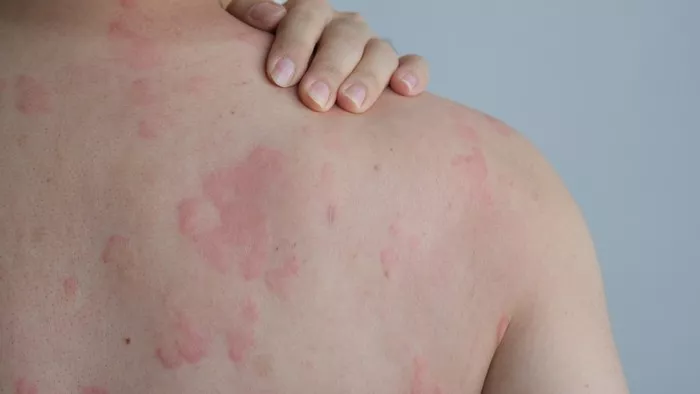
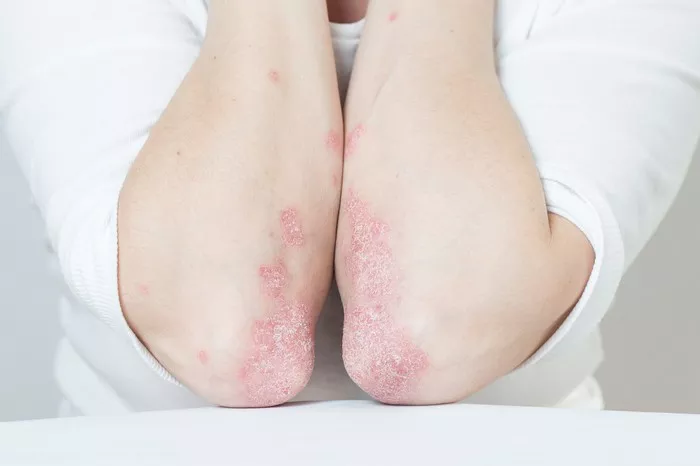
The U.S. Food and Drug Administration (FDA) has accepted the resubmission of a supplemental biologics license application (sBLA) for Dupixent (dupilumab), developed by Sanofi and Regeneron, for the treatment of chronic spontaneous urticaria (CSU). The application seeks approval for the therapy’s use in adults and pediatric patients aged 12 years and older whose CSU remains inadequately controlled by H1 antihistamines.
The FDA has set a target action date of April 18, 2025, for a decision on the application.
Dupixent is a fully human monoclonal antibody designed to inhibit the IL-4 and IL-13 pathways, which play a key role in type-2 inflammation. It is not classified as an immunosuppressant. Sanofi and Regeneron are jointly developing Dupixent under a global collaboration agreement.
The resubmitted application is based on data from the LIBERTY-CUPID Phase 3 clinical program, which includes Studies A, B, and C. Notably, the updated sBLA incorporates results from Study C, which involved patients with CSU who had not responded to standard antihistamine treatment.
Study C met both its primary and key secondary endpoints, further confirming the positive results seen in Study A. The findings indicated that Dupixent significantly reduced symptoms, including itch and hives, associated with CSU. The safety profile of Dupixent across the three studies was consistent with previous data, with the most common adverse events being injection site reactions and COVID-19 infections.
Studies A and C focused on patients with CSU uncontrolled by antihistamines, while Study B examined patients who were either refractory or intolerant to omalizumab. The program supports the clinical benefits of Dupixent in targeting type-2 inflammation, a key driver in several inflammatory and co-morbid conditions.
Dupixent has already been approved for CSU treatment in Japan and the UAE and is currently under review in the European Union. While its safety and efficacy for CSU outside these regions are still under evaluation, Dupixent has been approved in over 60 countries for various other indications, including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, and prurigo nodularis.
Additionally, the European Medicines Agency (EMA) recently approved Dupixent for the treatment of eosinophilic esophagitis in children aged one to 11 years who weigh at least 15 kg. This marks the first treatment approval for this condition in young children within the European Union, applicable to patients who are inadequately managed by or unsuitable for existing therapies.
Related topics: