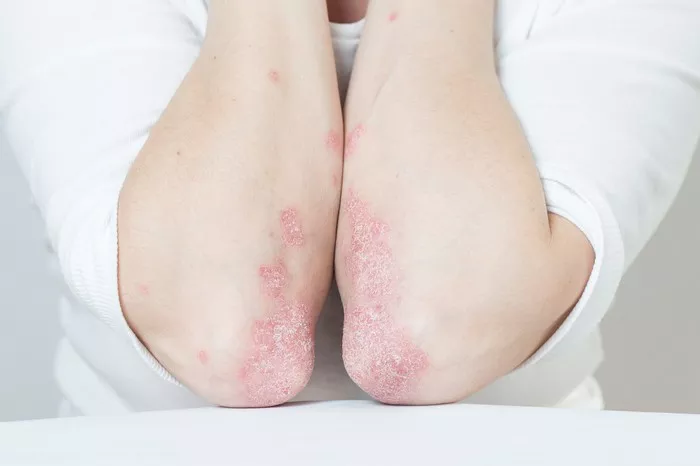
Alumis Inc., a biotechnology company based in South San Francisco, has announced a merger with Acelyrin Inc., a Southern California-based drug developer. The move is expected to extend the combined companies’ cash reserves well into 2027, just as they anticipate significant readouts from pivotal clinical trials in psoriasis and lupus.
Alumis, which trades under Nasdaq ticker ALMS, will also acquire Acelyrin’s drug, lonigutamab, developed for thyroid eye disease. However, the company indicated that it would reassess the program’s potential before advancing into late-stage trials, seeking to ensure it is differentiated and capital-efficient.
This merger marks the latest step in Alumis’s rapid growth trajectory. Formerly known as Esker Therapeutics Inc., the company has quickly established itself as a prominent player in the biotech sector, advancing its oral psoriasis drug, ESK-001, into a late-stage clinical trial. In addition to securing $250 million from venture and crossover investors, Alumis raised $193.3 million through its initial public offering (IPO) in June. The company has also begun enrolling patients in a mid-stage trial of ESK-001 for systemic lupus erythematosus (SLE), a severe form of lupus.
Looking ahead, Alumis plans to advance another drug, A-005, into a Phase II trial for multiple sclerosis patients later this year. In 2026, the company expects key data readouts, including topline results from the Phase III psoriasis trial, data from the Phase IIb SLE trial, and mid-stage data for A-005 in multiple sclerosis.
The merger strengthens Alumis’s financial position, which was bolstered by Acelyrin’s contribution of $448 million to the combined entity’s $737 million cash reserve. This infusion of capital will help the company continue advancing its pipeline amid a difficult biotech market, where many firms are scaling back or shedding programs to focus on immediate milestones. Alumis CEO Martin Babler emphasized the importance of scale and financial stability in today’s environment, noting that the merger provides the necessary resources to support the company’s ambitious goals.
The deal brings together two companies with divergent post-IPO paths. Acelyrin, which raised $540 million in a 2023 IPO, faced a setback when its lead drug failed in a Phase IIb/III trial for uveitis, an eye disease. In response, the company downsized its workforce by a third and streamlined its focus on lonigutamab.
The merger, slated to close in the second quarter, is structured as an all-stock transaction. Alumis shareholders will own 55% of the combined company, which will issue approximately 100 million shares. Acelyrin stockholders will receive 0.4274 shares of Alumis common stock for each of their existing shares.
Despite the fact that ESK-001’s clinical data won’t be available until next year, Alumis is already planning its commercial launch. To lead these efforts, the company appointed Jack Danilkowicz as Chief Commercial Officer. While the merger’s cash infusion won’t accelerate the launch, Babler stated that it provides flexibility in building the launch strategy.
ESK-001 targets tyrosine kinase 2 (TYK2), an enzyme involved in immune and inflammatory signaling pathways. This mechanism of action has shown promise in treating autoimmune diseases, where the immune system mistakenly attacks healthy cells. Although biologic therapies, which are typically more effective, dominate the treatment landscape, oral drugs like ESK-001 offer a more convenient alternative for patients, as large-molecule biologics can be difficult to administer.
Babler noted last year that approximately half of all psoriasis patients are treated with non-biologic drugs, highlighting the potential for ESK-001 to make an impact in the autoimmune space.
Related topics