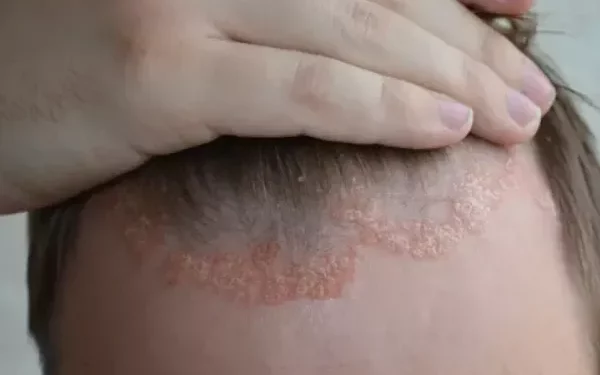
A recent study has highlighted the sustained benefits of tildrakizumab in treating scalp psoriasis, marking the first trial dedicated to showcasing the long-term efficacy of this anti-interleukin-23 inhibitor for the condition.
Scalp psoriasis, often challenging to treat, has limited options for patients, especially when topical therapies are ineffective or cosmetically unappealing. Dr. Howard L. Sofen, a clinical professor at UCLA’s David Geffen School of Medicine, and his team noted that tildrakizumab, an anti-IL-23 p19 antibody, is already approved for treating moderate to severe plaque psoriasis in adults who qualify for systemic therapy or phototherapy.
The study, a randomized, double-blind, placebo-controlled, phase 3b trial, specifically focused on the difficult-to-treat scalp area, which had not been evaluated in earlier tildrakizumab trials.
Participants were divided into two groups, receiving either tildrakizumab 100 mg (117 patients) or placebo (114 patients) during the study’s initial phase. The first treatment occurred at weeks 0 and 4. Those in the tildrakizumab group continued with 12-week dosing intervals through week 52, while the placebo group was switched to tildrakizumab at week 16, with additional doses at weeks 20, 32, and 44. A third part of the trial observed patients who discontinued treatment.
The primary analysis included 89 patients from the tildrakizumab group and 82 from the placebo group, with 22 discontinuing treatment from each group.
Researchers evaluated patients using a five-point IGA modified 2011 (scalp) score, considering treatment success as a score of 0 or 1 with at least a two-grade improvement, alongside a 90% or greater improvement in the PASI score.
By week 16, 49.4% of those initially treated with tildrakizumab achieved IGA success, compared to just 7.3% in the placebo group (P < .00001). PASI 90 response rates at week 16 were also significantly higher in the tildrakizumab group, at 60.7%, compared to only 4.9% in the placebo group (P < .00001).
At the 52-week mark, the proportion of tildrakizumab-treated patients achieving IGA success increased to 62.9%, while the placebo crossover group reached 56.1%. For the PASI 90 response, 65.2% of the tildrakizumab group and 57.3% of the crossover group showed improvements.
Additionally, tildrakizumab-treated patients reported a reduction in Scalp Itch-Numerical Scores from an average of 3.4 ± 2.8 at week 16 to 2.3 ± 2.6 at week 52.
Throughout the 72-week treatment period, treatment-emergent adverse events were reported in 53% of the tildrakizumab group and 51.8% of the placebo crossover group, with three and four serious events, respectively.
The researchers concluded that tildrakizumab effectively maintained both safety and efficacy in treating scalp psoriasis over 52 weeks, emphasizing the significance of this dedicated, long-term study with rigorous, clinically relevant outcomes.
Related topics