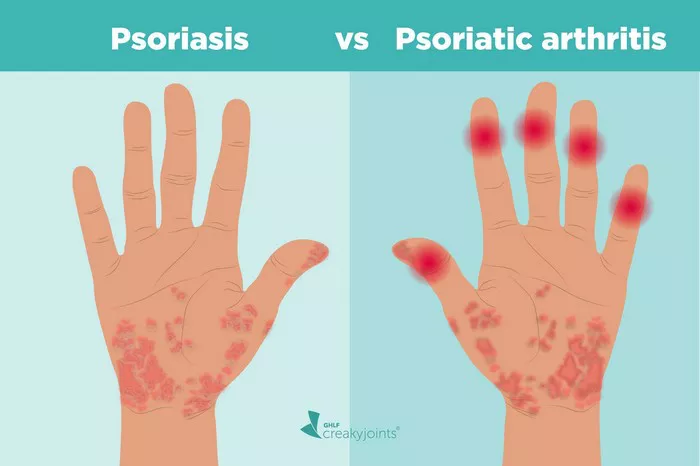
Psoriatic arthritis (PsA) stands as a chronic autoimmune condition characterized by inflammation of the joints and skin, affecting millions worldwide. The multifaceted nature of this disease demands a nuanced approach to treatment, and biologic therapies have emerged as a pivotal component in managing its symptoms effectively. However, with a plethora of biologic options available, determining the safest and most efficacious treatment regimen for PsA patients can be a daunting task for clinicians. In this article, we delve into the landscape of biologic therapies for PsA, exploring their safety profiles, efficacy, and factors to consider when selecting the optimal treatment strategy.
Understanding Psoriatic Arthritis and its Treatment Challenges
Before delving into the specifics of biologic therapies, it is essential to grasp the complexities of PsA and the challenges it poses for both patients and healthcare providers. PsA is a heterogeneous condition that manifests differently among individuals, with symptoms ranging from joint pain and stiffness to skin lesions and nail changes. The unpredictable nature of the disease progression coupled with its potential to cause irreversible joint damage underscores the importance of early and aggressive intervention.
Traditional disease-modifying antirheumatic drugs (DMARDs) and nonsteroidal anti-inflammatory drugs (NSAIDs) have historically been the mainstays of PsA treatment. However, their efficacy may be limited in many patients, particularly those with moderate to severe disease or inadequate response to conventional therapies. Biologic agents, which target specific components of the immune system implicated in PsA pathogenesis, have revolutionized the management of this condition, offering new hope for improved outcomes.
The Biologic Landscape: A Plethora of Choices
Biologic therapies for PsA primarily target proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-17 (IL-17), and interleukin-23 (IL-23), as well as other key players in the immune cascade. The array of biologics approved for PsA continues to expand, providing clinicians with a diverse armamentarium to tailor treatment according to individual patient needs.
TNF inhibitors, including adalimumab, etanercept, and infliximab, were among the first biologics approved for PsA and have demonstrated efficacy in reducing joint inflammation and improving clinical outcomes. However, concerns regarding safety, including the risk of serious infections and malignancies, have prompted the exploration of alternative biologic agents with potentially more favorable safety profiles.
IL-17 inhibitors, such as secukinumab and ixekizumab, have emerged as promising options for PsA treatment, targeting a different cytokine pathway implicated in disease pathogenesis. Clinical trials have shown significant improvements in joint symptoms, skin involvement, and quality of life with IL-17 inhibition, with some evidence suggesting a lower risk of serious adverse events compared to TNF inhibitors.
Similarly, IL-23 inhibitors, including ustekinumab and risankizumab, have demonstrated efficacy in PsA management, particularly in patients with concomitant psoriasis. By targeting IL-23, these biologics offer a unique mechanism of action that may provide sustained disease control and long-term remission.
Safety Considerations in Biologic Therapy Selection
While efficacy is undoubtedly a critical consideration in choosing a biologic therapy for PsA, safety considerations weigh heavily in the decision-making process. As with any immunomodulatory therapy, biologics carry inherent risks, including the potential for serious infections, malignancies, and autoimmune phenomena. Therefore, clinicians must carefully weigh the benefits of treatment against the potential risks, taking into account individual patient factors and comorbidities.
In recent years, comparative effectiveness studies and real-world evidence have provided valuable insights into the safety profiles of different biologic agents in PsA. While head-to-head trials are limited, indirect comparisons and post-marketing surveillance data offer valuable guidance for clinicians.
The Role of Real-World Evidence
Real-world evidence (RWE) plays an increasingly vital role in informing treatment decisions and evaluating the long-term safety and effectiveness of biologic therapies in PsA. By analyzing data from large-scale registries, electronic health records, and observational studies, researchers can gain valuable insights into treatment patterns, adverse events, and patient outcomes in real-world settings.
Recent RWE studies have shed light on the comparative safety profiles of biologic agents in PsA, helping to inform treatment decisions in clinical practice. For example, a retrospective cohort study comparing the risk of serious infections among biologic-naive PsA patients found no significant differences in infection rates between TNF inhibitors and IL-17 inhibitors, providing reassurance regarding the safety of both drug classes.
Similarly, post-marketing surveillance studies have identified rare but potentially serious adverse events associated with specific biologic therapies, such as the risk of reactivation of latent tuberculosis infection with TNF inhibitors. By systematically monitoring safety signals and adverse event reporting, regulatory agencies and healthcare providers can better understand the risks associated with biologic therapies and implement appropriate risk mitigation strategies.
Patient-Centered Care: Shared Decision-Making and Individualized Treatment
In the era of precision medicine, patient-centered care has become increasingly emphasized, with a focus on shared decision-making and individualized treatment approaches. When selecting a biologic therapy for PsA, clinicians must consider not only the efficacy and safety profile of the drug but also patient preferences, lifestyle factors, and treatment goals.
Engaging patients in shared decision-making empowers them to play an active role in their treatment journey, fostering a sense of ownership and collaboration. By discussing the potential benefits and risks of different biologic options in an open and transparent manner, clinicians can help patients make informed decisions that align with their values and preferences.
Furthermore, ongoing monitoring and reassessment are essential components of PsA management, allowing clinicians to evaluate treatment response, adjust therapy as needed, and address any emerging safety concerns. Regular follow-up visits provide an opportunity to assess disease activity, monitor for adverse events, and optimize treatment adherence, ultimately maximizing the likelihood of achieving favorable outcomes.
Conclusion
In conclusion, selecting the safest and most efficacious biologic therapy for PsA requires a thorough understanding of the disease pathophysiology, treatment landscape, and individual patient factors. While biologics have revolutionized the management of PsA, clinicians must carefully weigh the benefits and risks of treatment and engage patients in shared decision-making to optimize outcomes. Real-world evidence plays a crucial role in informing treatment decisions and evaluating the long-term safety and effectiveness of biologic therapies, providing valuable insights into their real-world performance. By embracing a patient-centered approach and leveraging the latest evidence-based practices, healthcare providers can strive to deliver personalized, effective, and safe care to PsA patients, ultimately improving their quality of life and long-term outcomes.