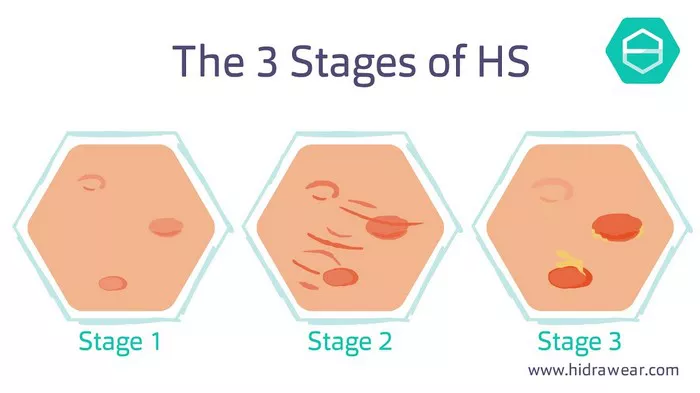
Hidradenitis Suppurativa (HS) is a chronic inflammatory skin condition characterized by painful nodules, abscesses, and sinus tracts primarily affecting the axillae, groin, and anogenital region. Despite its prevalence and impact on quality of life, the exact etiology of HS remains elusive. However, emerging research suggests a multifactorial interplay of genetic predisposition, immune dysregulation, and environmental factors in the pathogenesis of this debilitating disease.
Genetic Predisposition: Unlocking the Genetic Code
One of the primary factors contributing to the development of HS is genetic predisposition. Studies have identified a familial clustering of HS cases, indicating a hereditary component in its pathogenesis. Mutations in genes involved in innate immunity, such as the gamma-secretase complex genes (NCSTN, PSENEN, and PSEN1), have been implicated in HS development. These mutations disrupt the normal functioning of the immune system, leading to aberrant inflammatory responses within the skin.
Furthermore, genome-wide association studies (GWAS) have identified several susceptibility loci associated with HS, including genes related to the interleukin-1 (IL-1) pathway, tumor necrosis factor (TNF) signaling, and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway. Variations in these genes can disrupt immune homeostasis and predispose individuals to develop HS upon exposure to triggering factors.
Immune Dysregulation: Unraveling the Immunological Puzzle
The immune system plays a pivotal role in the pathogenesis of HS, with dysregulated immune responses driving the chronic inflammatory process observed in affected individuals. Several immune abnormalities have been identified in HS patients, including increased levels of pro-inflammatory cytokines such as interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-17 (IL-17).
Additionally, dysregulation of the innate immune system, particularly the aberrant activation of the NLRP3 inflammasome, has been implicated in the pathogenesis of HS. The NLRP3 inflammasome serves as a molecular platform for the activation of inflammatory caspases and the subsequent release of pro-inflammatory cytokines, perpetuating the inflammatory cascade observed in HS.
Moreover, alterations in the composition and function of the skin microbiome have been implicated in the pathogenesis of HS. Dysbiosis, characterized by an imbalance in the microbial community, can trigger aberrant immune responses and contribute to the development of inflammatory skin conditions, including HS.
Environmental Factors: Deciphering the External Triggers
While genetic predisposition and immune dysregulation play significant roles in HS pathogenesis, environmental factors also contribute to disease development and exacerbation. Several environmental factors have been implicated in HS, including obesity, smoking, hormonal changes, and mechanical stress.
Obesity is strongly associated with HS, with studies indicating a higher prevalence of the condition in individuals with a higher body mass index (BMI). Adipose tissue produces pro-inflammatory cytokines and adipokines, contributing to systemic inflammation and exacerbating the inflammatory milieu observed in HS.
Smoking has also been identified as a significant risk factor for HS development. Nicotine and other tobacco constituents can disrupt immune function and impair wound healing, exacerbating the inflammatory process in HS.
Hormonal changes, particularly fluctuations in sex hormones, have been implicated in HS pathogenesis. The condition often worsens during puberty and in women during menstruation, suggesting a hormonal influence on disease activity.
Furthermore, mechanical stress and friction in areas prone to HS lesions can exacerbate inflammation and contribute to disease progression. Constant rubbing and friction can damage the skin barrier, facilitating bacterial colonization and triggering inflammatory responses.
Conclusion
In conclusion, Hidradenitis Suppurativa is a complex inflammatory skin condition with a multifactorial etiology involving genetic predisposition, immune dysregulation, and environmental factors. While the exact cause of HS remains elusive, advancements in genetic and immunological research have shed light on the underlying mechanisms driving disease pathogenesis.
A comprehensive understanding of the interplay between genetic susceptibility, immune dysregulation, and environmental triggers is essential for developing targeted therapies and improving patient outcomes. Further research into the molecular pathways involved in HS development is warranted to unravel the complexities of this debilitating condition and pave the way for more effective treatment strategies.