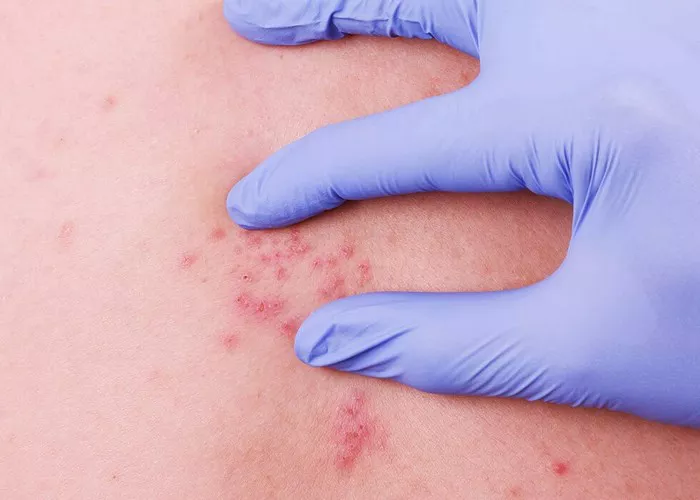
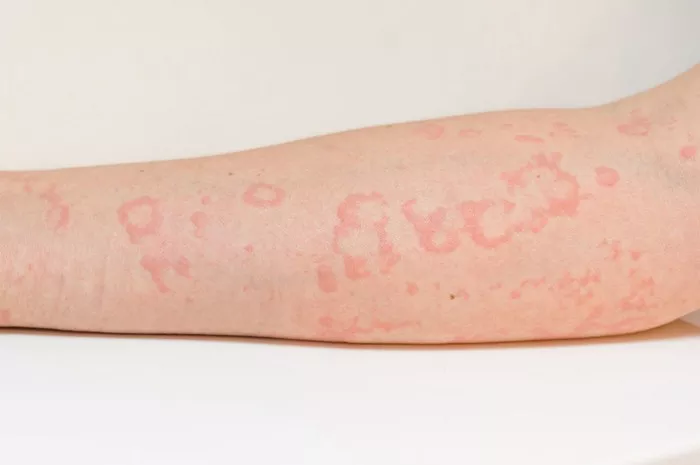
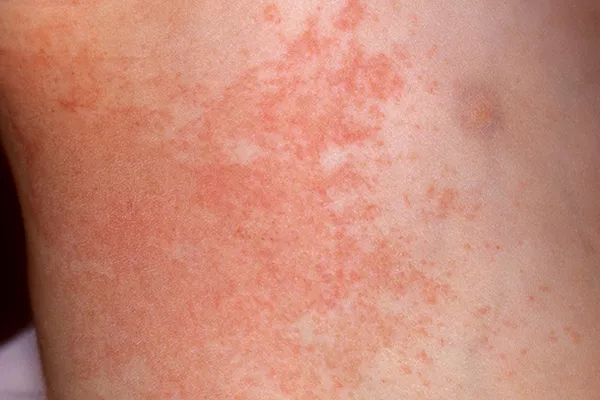
Herpes zoster, commonly known as shingles, is a viral infection characterized by a painful rash. While it primarily affects older adults and individuals with weakened immune systems, anyone who has had chickenpox can develop shingles. The causative agent behind this condition is the varicella-zoster virus (VZV), the same virus responsible for chickenpox. However, what triggers the reactivation of this dormant virus, leading to the development of herpes zoster, remains a subject of considerable scientific inquiry. In this article, we delve into the multifaceted factors contributing to the emergence of herpes zoster infection.
The Varicella-Zoster Virus: A Lingering Threat
Before delving into the triggers of herpes zoster, it’s essential to understand the varicella-zoster virus itself. VZV belongs to the herpesvirus family, known for its ability to establish lifelong latent infections in the human host. Upon initial infection, typically during childhood, VZV causes chickenpox (varicella). After recovery, the virus remains dormant in the sensory nerve ganglia, particularly the dorsal root ganglia, along the spinal cord and cranial nerve ganglia.
Reactivation: Unraveling the Dormant Threat
The transition from viral dormancy to reactivation is the crux of herpes zoster development. Several factors contribute to this process, which ultimately culminates in the manifestation of shingles.
1. Age: The Culprit Behind Immunosenescence
Age stands out as a significant risk factor for herpes zoster. As individuals grow older, their immune response undergoes a process known as immunosenescence. This age-related decline in immune function weakens the body’s ability to suppress latent viruses, such as VZV. Consequently, the virus can evade immune surveillance and reactivate, leading to the development of shingles. Studies have shown a sharp increase in herpes zoster incidence among individuals aged 50 and above, highlighting the pivotal role of age in this reactivation process.
2. Immune Compromise: A Breach in Defense
Individuals with compromised immune systems face an elevated risk of herpes zoster due to their impaired ability to control latent viral infections. Conditions such as HIV/AIDS, certain cancers, and immunosuppressive therapy post-organ transplantation weaken the immune response, providing an opportune environment for VZV reactivation. The compromised immune system fails to keep the virus in check, allowing it to travel along sensory nerve fibers and cause shingles. Thus, immune compromise emerges as a critical determinant in the pathogenesis of herpes zoster.
3. Stress: Unveiling the Mind-Body Connection
The intricate interplay between psychological stress and immune function has garnered considerable attention in the context of herpes zoster reactivation. Stress, whether acute or chronic, exerts profound effects on the immune system, compromising its ability to maintain viral latency. Elevated levels of stress hormones, such as cortisol, can disrupt the delicate balance between viral latency and reactivation, tipping the scales towards the latter. Furthermore, stress-induced immunosuppression creates an environment conducive to viral replication, fueling the emergence of shingles. While the exact mechanisms linking stress to herpes zoster remain under investigation, mounting evidence underscores the role of psychosocial factors in shaping disease susceptibility.
4. Physical Trauma: Nerves on Edge
Physical trauma or injury to sensory nerves can serve as a trigger for herpes zoster reactivation. Nerve damage disrupts the local microenvironment, facilitating the release of pro-inflammatory mediators and cytokines. This inflammatory milieu can awaken dormant VZV residing in the sensory ganglia, initiating the cascade of events leading to shingles. Common forms of nerve trauma include surgical procedures, accidents, and chronic conditions such as diabetes mellitus, which predispose individuals to neuropathy. Thus, while physical trauma alone may not directly cause herpes zoster, it can create a conducive environment for viral reactivation in susceptible individuals.
5. Genetic Predisposition: Unraveling the Role of Genetics
Genetic factors play a significant role in determining an individual’s susceptibility to herpes zoster. Variations in immune-related genes, particularly those involved in innate and adaptive immunity, can influence the likelihood of viral reactivation. Polymorphisms in genes encoding cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), have been implicated in modulating host response to VZV infection. Additionally, genetic differences in viral-host interactions may influence the establishment and maintenance of viral latency, thereby influencing the risk of developing shingles. While further research is needed to elucidate the precise genetic mechanisms underlying herpes zoster susceptibility, genetic predisposition undoubtedly contributes to the complex interplay of factors shaping disease outcome.
Conclusion
Herpes zoster infection, though often perceived as a mere complication of childhood chickenpox, is a complex manifestation of varicella-zoster virus reactivation. From age-related immune decline to the intricate interplay of psychosocial factors, numerous determinants converge to precipitate the emergence of shingles. By unraveling the underlying causes of herpes zoster, researchers strive not only to enhance our understanding of viral pathogenesis but also to develop targeted strategies for prevention and management. As we continue to explore the intricate nexus of host-virus interactions, the quest for effective interventions against herpes zoster remains a pressing imperative in public health discourse.