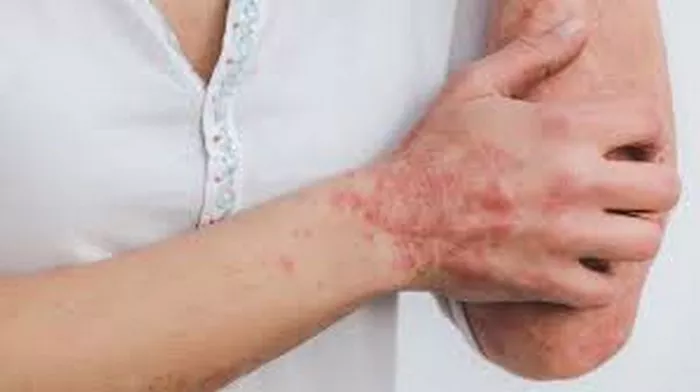
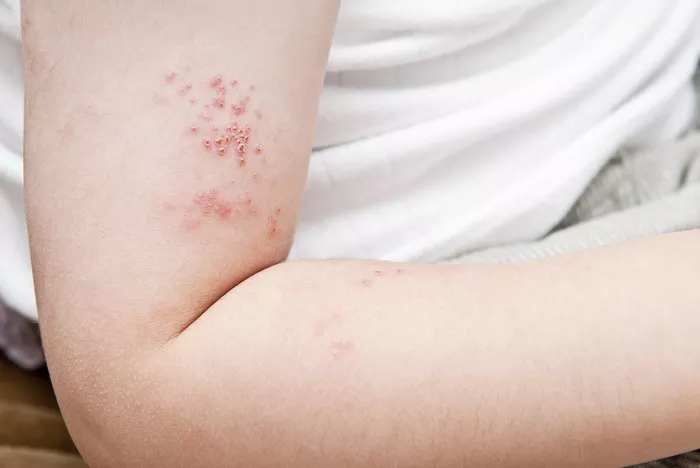
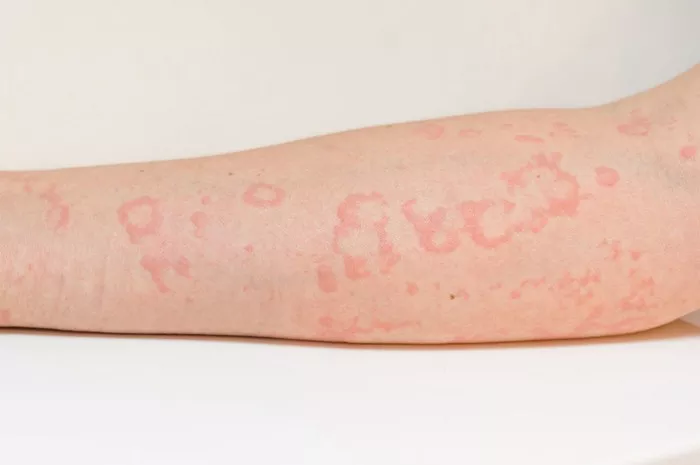
Psoriasis is a chronic inflammatory skin condition affecting millions of individuals worldwide. It is characterized by the presence of red, scaly patches on the skin, often accompanied by itching, pain, and in severe cases, joint inflammation. For decades, researchers have sought to unravel the complex mechanisms underlying this debilitating disease, particularly in terms of whether it primarily stems from autoimmune or autoinflammatory processes.
The debate surrounding the etiology of psoriasis has been ongoing, with evidence suggesting involvement of both autoimmune and autoinflammatory components. To comprehend this intricate interplay, it is essential to delve into the pathophysiology of psoriasis and explore the molecular mechanisms driving its development and progression.
Psoriasis: A Multifactorial Disorder
Psoriasis is widely regarded as a multifactorial disorder, influenced by genetic predisposition, environmental triggers, and dysregulated immune responses. Central to its pathogenesis is the dysregulation of the immune system, leading to aberrant activation of immune cells and subsequent inflammation within the skin.
The Autoimmune Perspective
Historically, psoriasis has been classified as an autoimmune disease, characterized by the immune system’s misguided attack on healthy tissues. According to this viewpoint, autoreactive T cells, particularly Th1 and Th17 cells, play a pivotal role in orchestrating the inflammatory cascade observed in psoriatic lesions.
In autoimmune psoriasis, the immune system mistakenly identifies self-antigens, possibly derived from keratinocytes or other skin components, as foreign invaders, triggering an inflammatory response. This dysregulated immune response leads to the release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-17 (IL-17), and interleukin-23 (IL-23), which contribute to the characteristic features of psoriatic plaques.
Furthermore, genetic studies have identified several susceptibility loci associated with psoriasis, including genes encoding human leukocyte antigens (HLAs) and cytokines involved in immune regulation. These findings underscore the genetic component of psoriasis and support its classification as an autoimmune disorder.
The Autoinflammatory Paradigm
On the other hand, the autoinflammatory perspective posits that psoriasis primarily arises from dysregulated innate immune responses, independent of adaptive immune mechanisms. Unlike autoimmune diseases, which involve the recognition of self-antigens by adaptive immune cells, autoinflammatory disorders are characterized by innate immune dysfunction, leading to unprovoked inflammation.
In psoriasis, innate immune cells, such as dendritic cells, macrophages, and neutrophils, are believed to play a central role in driving inflammation within the skin. These cells produce pro-inflammatory mediators, including interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), which contribute to the pathogenesis of psoriatic lesions.
Moreover, recent studies have highlighted the role of the inflammasome, a multiprotein complex involved in innate immune signaling, in the pathophysiology of psoriasis. Activation of the inflammasome leads to the production of pro-inflammatory cytokines and amplifies the inflammatory response in psoriatic skin.
The Interplay Between Autoimmunity and Autoinflammation
While the autoimmune and autoinflammatory paradigms offer valuable insights into the pathophysiology of psoriasis, mounting evidence suggests that the distinction between these two mechanisms may not be clear-cut. Instead, psoriasis may represent a spectrum disorder, encompassing elements of both autoimmunity and autoinflammation.
Recent studies have highlighted the crosstalk between adaptive and innate immune pathways in psoriasis, indicating a complex interplay between T cells, dendritic cells, and keratinocytes in driving disease pathology. For instance, autoreactive T cells not only contribute to the adaptive immune response but also stimulate the production of pro-inflammatory cytokines by innate immune cells, thereby perpetuating inflammation within the skin.
Furthermore, the presence of autoantibodies targeting skin components, such as keratin and filaggrin, in psoriatic patients suggests a potential autoimmune component in disease pathogenesis. These autoantibodies may arise secondary to tissue damage and contribute to the perpetuation of inflammation in psoriatic lesions.
Therapeutic Implications
The evolving understanding of psoriasis as a complex interplay between autoimmunity and autoinflammation has significant implications for the development of targeted therapies. While current treatments primarily focus on suppressing inflammation, future therapeutic approaches may aim to modulate both adaptive and innate immune pathways involved in psoriasis pathogenesis.
Biologic agents targeting specific cytokines, such as TNF-α, IL-17, and IL-23, have revolutionized the management of psoriasis by effectively dampening the inflammatory response. However, the heterogeneity of psoriasis phenotypes and treatment responses underscores the need for personalized therapeutic strategies tailored to individual patients.
Additionally, emerging therapies targeting innate immune pathways, such as the inflammasome, hold promise in addressing the underlying mechanisms driving inflammation in psoriasis. By targeting key mediators of innate immune activation, these novel treatments may offer alternative therapeutic options for patients with refractory disease.
Conclusion
In conclusion, the pathophysiology of psoriasis is characterized by a complex interplay between autoimmune and autoinflammatory processes. While traditional paradigms have focused on either adaptive or innate immune mechanisms, emerging evidence suggests that psoriasis represents a spectrum disorder, encompassing elements of both autoimmunity and autoinflammation.
Understanding the intricate molecular pathways driving inflammation in psoriasis is crucial for the development of targeted therapeutic interventions aimed at modulating immune dysregulation. By elucidating the underlying mechanisms of disease pathogenesis, researchers can pave the way for the development of more effective and personalized treatments for patients with psoriasis.
Related Topics:
Psoriasis vs Plaque Psoriasis: Understanding the Differences