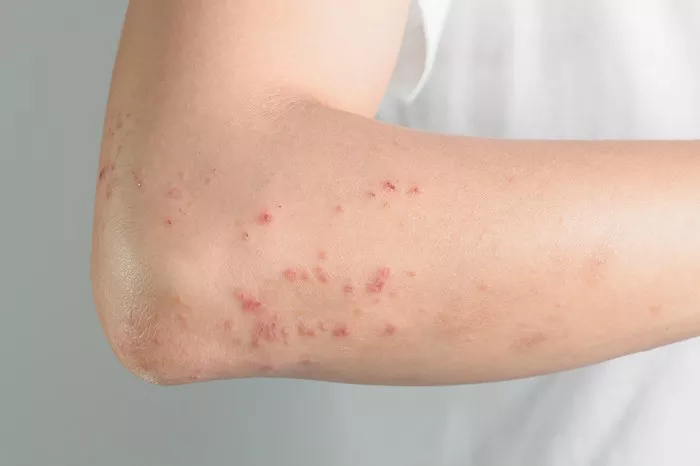
A recent study has revealed that GSK’s shingles vaccine, Shingrix, remains highly effective in preventing shingles for more than a decade among adults aged 50 and over.
The study’s results, presented at a medical conference, stem from a long-term follow-up trial that monitored participants for up to 11 years post-vaccination. Last year, GlaxoSmithKline Pharmaceuticals introduced Shingrix (Zoster Vaccine Recombinant, Adjuvanted) in India to combat shingles (herpes zoster) and post-herpetic neuralgia in individuals aged 50 years and above.
Shingles is a painful rash caused by the reactivation of the varicella-zoster virus, the same virus responsible for chickenpox. Approximately one in three individuals will develop shingles during their lifetime, with risk increasing with age.
Shingrix is administered as a two-dose regimen and has demonstrated efficacy in preventing shingles and its complications, such as post-herpetic neuralgia (PHN), a lingering nerve pain following shingles.
The new study, named ZOSTER-049, engaged over 7,000 participants across 18 countries. Results indicated sustained high efficacy of the vaccine throughout the 11-year follow-up period, with an overall efficacy rate of 79.7% in adults aged 50 and over from six to 11 years after vaccination.
Rashmi Hegde, Executive Vice President of Medical Affairs at GSK India, remarked, “These findings, demonstrating enduring efficacy and protection against shingles over an extensive 11-year duration, offer reassurance for healthcare providers and patients alike. Given the heightened risk of shingles complications in aging adults, we hope these results inspire policymakers to include shingles vaccination in adult public immunization plans.”
Shingles presents a significant global health concern, impacting up to one in three individuals throughout their lives. Factors like advancing age and immunodeficiency heighten the risk of shingles, with complications including post-herpetic neuralgia (PHN), a debilitating nerve pain.
The study also confirmed the vaccine’s safety and tolerability, with no new safety issues identified during the follow-up period. The most commonly reported side effects included pain at the injection site, muscle aches, fatigue, and headache, typically mild to moderate and short-lived.
Researchers continue to analyze long-term data on Shingrix and will conduct further investigations to assess the necessity of revaccination in the future.