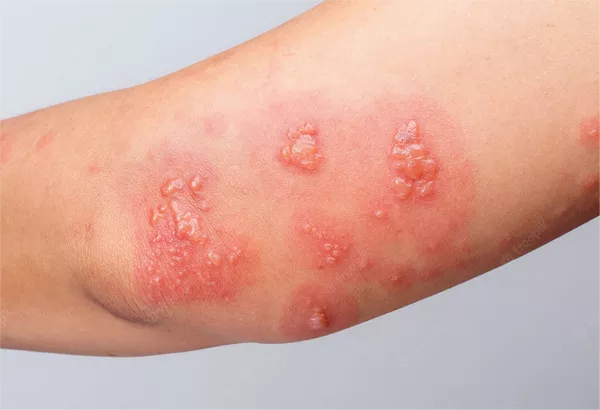
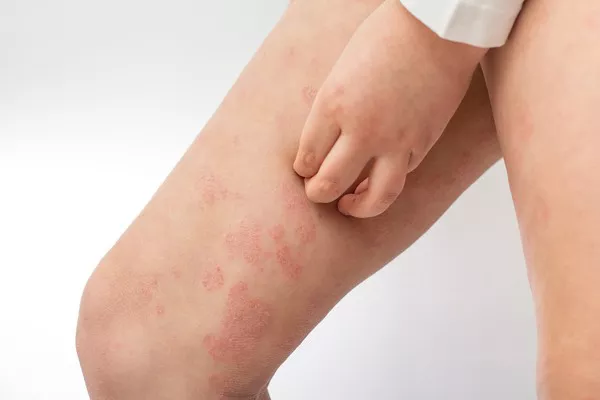
MoonLake Immunotherapeutics has announced the commencement of patient screenings at a US site for its global phase 3 trial, VELA. This trial aims to assess the safety and efficacy of sonelokimab for treating moderate to severe hidradenitis suppurativa (HS). Building on the positive outcomes of the phase 2 MIRA trial, the phase 3 VELA trial will enroll 800 patients across two identical studies, VELA-1 (NCT06411899) and VELA-2 (NCT06411379).
The VELA trials will compare a single 120mg dose of sonelokimab to a placebo, using the Hidradenitis Suppurativa Clinical Response (HiSCR) 75 as the primary endpoint. HiSCR75 is defined as a 75% or greater reduction in the total count of abscesses and inflammatory nodules without any increase in abscesses or draining tunnels at week 16. Notably, VELA is the first phase 3 HS trial to use HiSCR75 as its primary endpoint.
Secondary endpoints include the proportion of patients achieving HiSCR50, changes from baseline in the International Hidradenitis Suppurativa Severity Score System, a reduction of four or more in the Dermatology Life Quality Index, a 50% or greater reduction in the Numerical Rating Scale for the Patient’s Global Assessment of Skin Pain, and the complete resolution of draining tunnels.
After the initial 16 weeks, all participants will receive 120mg of sonelokimab through week 52, followed by an open-label extension for up to two years. The trial design mirrors the phase 2 MIRA trial (NCT05322473), which determined the optimal dose of sonelokimab for HS. Primary endpoint data from week 16 of the VELA trial is expected in mid-2025.
Dr. Hadar Lev-Tov, an associate professor of clinical dermatology and cutaneous surgery at the Miller School of Medicine in Miami and president of the Hidradenitis Suppurativa Foundation, emphasized the significance of the phase 3 program. “HS is a chronic inflammatory skin condition with a range of debilitating symptoms including pain, malodorous drainage, low mood, and depression. With only two FDA-approved biologics, there is an urgent need for new treatment options that treat all patient types and lesions, with the opportunity for inflammatory remission. The unique characteristics and mode of action of MoonLake’s Nanobody, sonelokimab, to effectively inhibit IL-17F in addition to IL-17A in deep tissue inflammation has to date shown promising outcomes, highlighting the importance of this phase 3 program, and placing HS at the forefront of dermatological innovation,” Lev-Tov stated in a news release.
The phase 3 trial’s progress follows positive feedback from the US Food and Drug Administration and the European Medicines Agency, who expressed support for MoonLake’s phase 3 program evaluating sonelokimab.
Dr. Seth B. Forman, principal investigator at CenExel-FCR, shared his enthusiasm for the VELA program. “It is with great enthusiasm that I am participating as an investigator in the Phase 3 VELA program investigating the Nanobody sonelokimab for HS, signifying a substantial advancement in addressing the critical unmet need for more treatment options for individuals living with HS. As a physician, I witness first-hand the immense demand for novel treatment options for people living with HS, particularly those that can achieve elevated response thresholds (eg, HiSCR75 and beyond). The findings from the phase 2 MIRA trial offer valuable insights into what may be possible as we work with our patients to establish more ambitious treatment goals and alleviate the disease burden of this debilitating condition,” Forman said in a news release.
Last month, MoonLake announced a partnership with Komodo Health to advance research and treatments for inflammatory skin and joint diseases, such as HS, psoriasis, and psoriatic arthritis. This collaboration aims to enhance the impact of sonelokimab on diseases with significant unmet patient needs.
Additional Indications
MoonLake is also exploring sonelokimab for treating psoriatic arthritis in its phase 2 ARGO trial (NCT05640245), with plans to advance to phase 3 trials in the fourth quarter of 2024. Phase 2 results showed significant improvements in all key endpoints, with approximately 60% of patients achieving an American College of Rheumatology 50 response and Minimal Disease Activity at week 24.
Sonelokimab has been evaluated in a phase 2b trial (NCT03384745) involving 313 patients with moderate to severe plaque-type psoriasis, showing high threshold clinical responses and a safety profile similar to the active control, secukinumab.