Scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, have developed a new over-the-counter topical probiotic for eczema. This breakthrough, which leverages the bacteria Roseomonas mucosa naturally found on healthy skin, promises to alleviate symptoms for both adults and children. The probiotic, now commercially available, represents a significant advancement following seven years of research in NIAID’s Laboratory of Clinical Immunology and Microbiology (LCIM).
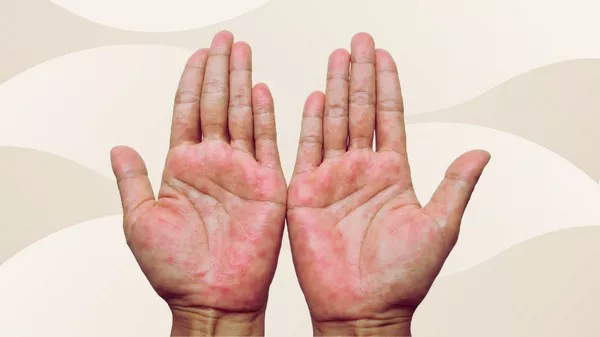
Eczema, also known as atopic dermatitis, is a chronic inflammatory skin condition affecting around 20% of children and 10% of adults globally. It is characterized by dry, itchy skin that can weaken the skin’s barrier, making it susceptible to infections. The presence of R. mucosa, a naturally occurring bacterium in the skin microbiome, can help restore the balance of skin lipids, which are often deficient in eczema sufferers.
Under the leadership of Dr. Ian Myles, chief of the LCIM Epithelial Research Unit, NIAID researchers identified specific strains of R. mucosa that significantly reduce eczema-related inflammation and enhance the skin’s natural barrier. This discovery emerged from comprehensive research, including laboratory, animal, and human studies. The resultant probiotic, formulated by Skinesa and branded as Defensin, is now available for non-therapeutic use.
Clinical trials, including Phase 1/2 open-label and Phase 2 blinded, placebo-controlled studies, showed promising results. Participants experienced over 75% improvement in eczema severity across various body parts, such as the inner elbows, knees, hands, trunk, and neck. The probiotic also improved skin barrier function, reduced the need for corticosteroids, alleviated itching, and enhanced overall quality of life. Remarkably, the therapeutic effects persisted for up to eight months post-treatment.
Looking ahead, NIAID plans to conduct additional clinical trials to further validate the efficacy of R. mucosa in reducing eczema symptoms. The findings, expected in 2024, could support an application to the Food and Drug Administration (FDA) for regulation as a nonprescription drug, potentially making the treatment widely accessible to those suffering from eczema.
This innovative approach to eczema treatment not only offers new hope to sufferers but also exemplifies the potential of microbiome-based therapies in managing chronic skin conditions.
Related Topics: