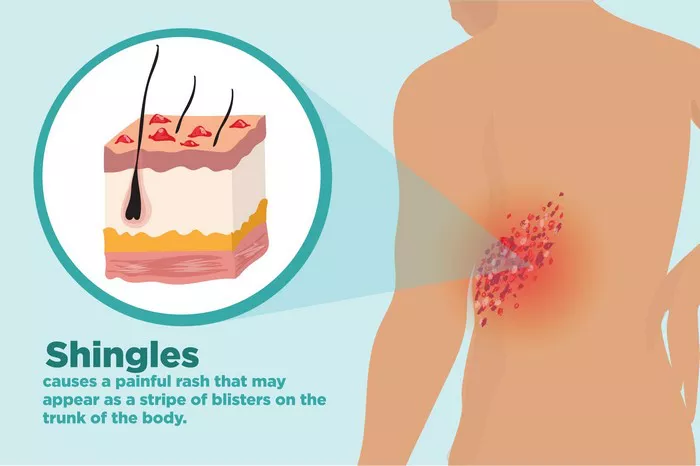
Shingrix is a widely used vaccine designed to protect against shingles, a painful condition caused by the reactivation of the varicella-zoster virus, the same virus responsible for chickenpox. This vaccine has significantly improved the prevention of shingles and its complications, particularly in older adults. However, like all medical interventions, Shingrix is not without potential side effects. One area of concern that has garnered attention is the possibility of Shingrix causing neuropathy. This article aims to explore the relationship between Shingrix and neuropathy, delving into what neuropathy is, how vaccines can potentially cause it, and the specific evidence related to Shingrix.
Understanding Neuropathy
Neuropathy, or peripheral neuropathy, refers to damage to the peripheral nerves, which can result in pain, numbness, tingling, and weakness, typically in the hands and feet. There are various types of neuropathy, with causes ranging from diabetes, infections, and autoimmune diseases to traumatic injuries and exposure to toxins. The symptoms of neuropathy can vary in severity and can significantly impact an individual’s quality of life.
Vaccine-Induced Neuropathy: An Overview
Vaccines are designed to stimulate the immune system to protect against specific infections. While they are generally safe and effective, vaccines can occasionally cause side effects. These can range from mild, such as pain at the injection site, to more severe reactions, including neurological complications. Vaccine-induced neuropathy is a rare but recognized phenomenon. It occurs when the immune response triggered by the vaccine causes inflammation or damage to the nerves.
Mechanisms of Vaccine-Induced Neuropathy
The exact mechanisms by which vaccines may cause neuropathy are not fully understood, but several hypotheses exist:
- Autoimmune Reaction: Vaccines may trigger an autoimmune response where the body’s immune system mistakenly attacks its own nerves, leading to neuropathy.
- Molecular Mimicry: Some components of vaccines might resemble nerve tissues, causing the immune system to target both the vaccine and the nerves.
- Direct Toxicity: Adjuvants or preservatives in vaccines might have a direct toxic effect on nerve cells.
- Inflammatory Response: The general inflammatory response to a vaccine could cause inflammation in or around the nerves, leading to neuropathy.
Shingrix and Neuropathy: Current Evidence
Shingrix, approved by the FDA in 2017, is a recombinant vaccine that contains a non-live antigen to prevent shingles. Since its introduction, it has been highly effective, reducing the risk of shingles by over 90% in older adults. Despite its benefits, reports of adverse effects, including neuropathy, have emerged.
Clinical Trials and Initial Reports
During the clinical trials of Shingrix, the most common side effects reported were mild to moderate, such as pain at the injection site, fatigue, muscle pain, and headache. Serious adverse effects were rare. However, post-marketing surveillance and anecdotal reports have indicated instances of neuropathy following vaccination.
Case Reports and Observational Studies
Several case reports have documented individuals developing neuropathy after receiving Shingrix. These reports describe symptoms such as numbness, tingling, and pain in the extremities. While these cases suggest a potential link, they do not establish causation. Observational studies have also been conducted to assess the incidence of neuropathy in vaccinated populations compared to unvaccinated groups. Some studies have found a slightly increased risk of neuropathy following vaccination, while others have not found a significant association.
Mechanistic Insights
Researchers have investigated the possible mechanisms by which Shingrix might cause neuropathy. One hypothesis is that the immune response elicited by the vaccine could lead to an autoimmune reaction targeting peripheral nerves. Additionally, the adjuvant used in Shingrix, AS01B, designed to enhance the immune response, might contribute to the development of neuropathy in susceptible individuals.
Assessing the Risk: Shingrix Versus Other Vaccines
It is important to contextualize the risk of neuropathy with Shingrix in comparison to other vaccines. Neuropathy has been reported as a rare side effect for several vaccines, including those for influenza and hepatitis B. The overall risk of developing neuropathy following vaccination remains low. For Shingrix, the benefits of preventing shingles, a condition that can itself cause severe nerve pain and long-term complications, generally outweigh the risks of rare adverse effects like neuropathy.
SEE ALSO: Shingles vs. Cold Sores: Understanding the Differences
Risk Factors for Neuropathy with Shingrix
Certain individuals may be at a higher risk of developing neuropathy following Shingrix vaccination. These risk factors include:
- Pre-existing Neuropathy: Individuals with existing peripheral neuropathy may be more susceptible to exacerbation or recurrence of symptoms.
- Autoimmune Disorders: Those with autoimmune conditions might have an increased risk due to their already heightened immune activity.
- Genetic Predisposition: Genetic factors could make some individuals more prone to developing neuropathy after vaccination.
Recommendations for Patients and Healthcare Providers
Given the potential risk of neuropathy with Shingrix, it is crucial for healthcare providers to thoroughly assess patients before vaccination. Patients with a history of neuropathy or autoimmune disorders should discuss the risks and benefits with their healthcare provider. Additionally, monitoring for symptoms of neuropathy following vaccination is essential for early detection and management.
For Patients
- Inform Your Healthcare Provider: If you have a history of neuropathy or autoimmune disease, inform your provider before receiving Shingrix.
- Monitor Symptoms: Pay attention to any new or worsening symptoms of neuropathy after vaccination, such as numbness, tingling, or pain in the extremities.
- Report Adverse Effects: Report any adverse effects to your healthcare provider and the Vaccine Adverse Event Reporting System (VAERS).
For Healthcare Providers
- Assess Risk Factors: Evaluate patients for risk factors of neuropathy before administering Shingrix.
- Educate Patients: Inform patients about the potential risks and benefits of the vaccine, especially those with pre-existing conditions.
- Monitor and Report: Monitor patients for symptoms of neuropathy post-vaccination and report any adverse effects to VAERS.
Conclusion
The question of whether Shingrix can cause neuropathy is complex and multifaceted. While there have been reports and some evidence suggesting a potential link, the overall risk appears to be low. The benefits of Shingrix in preventing shingles, which can itself cause severe nerve pain and complications, generally outweigh the risks of rare side effects like neuropathy. Further research is needed to fully understand the mechanisms and risk factors associated with vaccine-induced neuropathy. In the meantime, healthcare providers and patients should engage in informed discussions to make the best decisions based on individual risk profiles.
Related Topics: