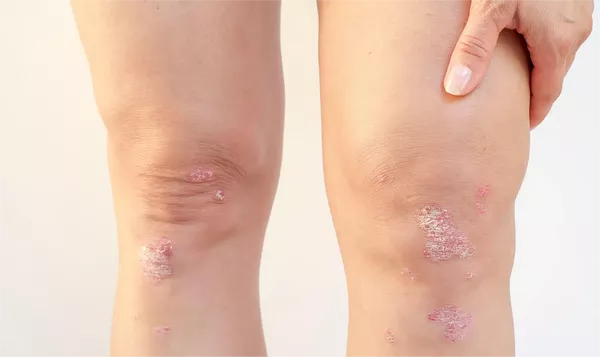
At the World Psoriasis & Psoriatic Arthritis (IFPA) Conference in Stockholm, Sweden, AbbVie presented promising new data from a 148-week interim analysis of the ongoing VALUE trial. This extensive, multi-country, prospective post-marketing observational study evaluates the effectiveness of risankizumab in treating moderate-to-severe psoriasis in real-world settings. The findings underscore risankizumab’s superior and durable clinical responses, particularly in achieving and maintaining significant skin clearance compared to other biologics.
Study Overview
The VALUE trial involves over 2,600 patients, and the interim analysis has shown that those treated with risankizumab experience complete or near-complete skin clearance at notably higher rates than those treated with other biologics. Specifically, 67.2% of risankizumab patients achieved PASI 90 (90% reduction in the Psoriasis Area and Severity Index) compared to 45.3% in the other biologics cohort. Furthermore, 55.2% of risankizumab patients reached PASI 100 (complete clearance) versus 34.6% of those on other treatments.
Patients on risankizumab also reported less frequent treatment changes and higher Treatment Satisfaction Questionnaire for Medication (TSQM) scores, indicating a better overall patient experience.
Expert Insights
In a recent Q&A with Dermatology Times, Dr. Diamant Thaçi, a university professor at the University of Lübeck in Germany and a specialist in chronic inflammatory skin diseases, provided deeper insights into the VALUE study and its implications for clinical practice.
Q&A with Dr. Diamant Thaçi
Q: Can you provide a brief overview of the VALUE study, its primary objectives, and findings?
A: The VALUE study stands out because it reflects real-world practice rather than the controlled environment of clinical trials. Real-world data is crucial as it includes a broader patient population, including those who may not meet the strict criteria of clinical trials. The study aimed to evaluate risankizumab’s effectiveness in everyday clinical settings, comparing it with other biologics. The interim results, covering three years of data, show that risankizumab provides consistent and superior skin clearance and patient satisfaction compared to other biologics, involving around 2,000 patients on risankizumab and nearly 900 on other biologics.
Q: What were the key challenges faced during the execution of this real-world study, and how were they addressed?
A: Real-world studies face unique challenges such as varying healthcare systems, patient histories, and insurance issues. Unlike clinical trials, which often include biologic-naive patients without systemic treatment history, real-world studies must account for previous treatments, eligibility, and insurance coverage. We addressed these by ensuring the study design mirrored daily clinical practice, prescribing treatments according to local labels and regulations, thus capturing a more accurate reflection of everyday challenges and outcomes.
Q: How did risankizumab perform in comparison to other biologics in terms of long-term clinical responses and treatment durability?
A: Risankizumab has demonstrated exceptional treatment durability, aligning with the known performance of IL-23 inhibitors. The real-world data confirmed that patients maintained their responses longer with risankizumab compared to other biologics, consistent with clinical trial observations. The study also highlighted that patients on other biologics often experienced a gradual loss of response over time, emphasizing risankizumab’s stability and efficacy in real-world settings.
Q: What insights do the DLQI and TSQM scores provide about the patient experience and satisfaction with risankizumab treatment?
A: The DLQI (Dermatology Life Quality Index) and TSQM scores reveal significant improvements in patients’ quality of life and satisfaction with risankizumab. The real-world setting showed that patients quickly switched to effective treatments without washout periods, achieving high PASI scores rapidly. The long-term treatment led to sustained patient satisfaction, reflected in stable or improving DLQI scores, even as skin symptoms improved. This underscores the importance of effective, durable treatments in enhancing patients’ overall well-being.
Q: With the study ongoing, what are the next steps for the VALUE trial, and what additional data are you looking forward to collecting?
A: We aim to gather more data on patient-reported outcomes, work productivity, and the safety profiles of different treatments. Understanding how treatments like risankizumab impact daily life and work is crucial. Additionally, monitoring safety in real-world settings, especially for patients with comorbidities, will provide valuable insights into the long-term viability of these treatments. We are particularly interested in confirming the safety profiles observed in clinical trials and identifying any new signals that may emerge.
Q: How might these interim findings influence clinical practice and the management of moderate-to-severe psoriasis?
A: The findings solidify risankizumab’s position as a highly effective treatment option for moderate-to-severe psoriasis. The VALUE study reinforces the drug’s efficacy and durability in real-world settings, suggesting that clinicians might consider risankizumab as a first-line treatment rather than reserving it for later stages. This approach could reduce the need for multiple treatment switches, improving patient outcomes and satisfaction. The data also highlight the importance of assessing work productivity and quality of life in treatment decisions, offering a more holistic view of patient care.
Q: Is there anything else that you would like to share with dermatology clinicians?
A: It’s vital for clinicians to aim for high improvement rates in patients’ skin conditions. The VALUE study’s real-world data is particularly relevant for practitioners, providing actionable insights to guide treatment decisions. These findings can help clinicians make informed choices about biologic treatments, potentially leading to better patient outcomes and higher satisfaction rates in daily practice. The ultimate goal is to offer treatments that not only improve clinical symptoms but also enhance patients’ quality of life.
Related Topics: