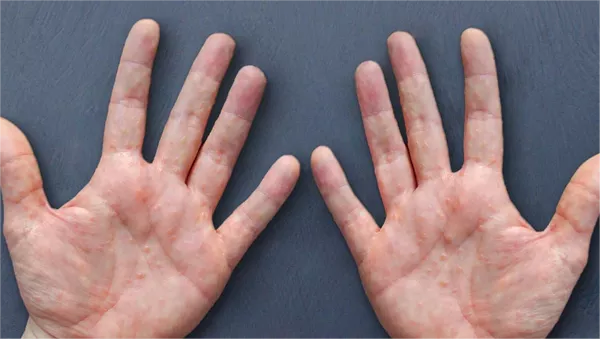
BALLERUP, Denmark, July 26, 2024 — LEO Pharma A/S, a prominent leader in medical dermatology, has announced a favorable opinion from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP). The recommendation advocates for the approval of Anzupgo® (delgocitinib cream) as a treatment for adults with moderate to severe chronic hand eczema (CHE) where topical corticosteroids are either insufficient or unsuitable.
Delgocitinib cream, a novel topical pan-JAK inhibitor, addresses CHE by targeting the JAK-STAT signaling pathway, which is critical in the development of the condition. Currently, no topical treatments are specifically approved for adults with moderate to severe CHE when traditional corticosteroids are not effective.
Chronic hand eczema is a complex, inflammatory skin disorder marked by itching and pain, with pathophysiology involving skin barrier dysfunction, inflammation, and changes in the skin microbiome. This condition significantly affects the psychological, social, and occupational well-being of patients.
Christophe Bourdon, Chief Executive Officer of LEO Pharma, commented on the milestone: “CHE poses considerable challenges to those affected, impacting personal and professional lives. With limited existing treatment options, we are committed to improving care standards for individuals suffering from CHE. Today’s positive opinion brings us closer to addressing this critical need.”
The CHMP’s endorsement is based on data from the Phase 3 DELTA 1 and DELTA 2 trials, which assessed delgocitinib cream’s safety and efficacy compared to a cream vehicle. Both studies achieved their primary and secondary endpoints, demonstrating the cream’s effectiveness. Participants who completed these trials were invited to join the 36-week DELTA 3 open-label extension trial, which confirmed the cream’s long-term safety.
Kreesten Meldgaard Madsen, Chief Development Officer of LEO Pharma, highlighted the impact of CHE: “The visible and burdensome nature of CHE affects daily life and emotional well-being. We are proud of this advancement and remain focused on supporting those with CHE.”
The positive CHMP opinion will be reviewed by the European Commission (EC). If approved, delgocitinib cream will be authorized for use across EU member states, as well as Iceland, Norway, and Liechtenstein. Regulatory submissions in other regions are also underway.
About the DELTA Trials
The DELTA 1 and DELTA 2 trials were randomized, double-blind, vehicle-controlled studies designed to evaluate the efficacy of delgocitinib cream. The primary endpoint was treatment success at Week 16, defined as an Investigator’s Global Assessment for chronic hand eczema (IGA-CHE) score of 0 or 1, reflecting clear or almost clear skin with a significant improvement from baseline. Secondary endpoints included reductions in itch and pain scores and improvements in the Hand Eczema Severity Index (HECSI).
Participants who completed the 16-week treatment phase were offered the opportunity to continue in the DELTA 3 open-label extension trial to assess long-term safety.
About Chronic Hand Eczema
Chronic hand eczema (CHE) is characterized by eczema lasting over three months or recurring multiple times within a year. It is the most prevalent hand skin disorder, affecting approximately 9% of people annually. CHE is marked by fluctuating symptoms including itching, pain, erythema, scaling, and fissures, which impose significant psychological and functional burdens.
About Anzupgo® (delgocitinib cream)
Anzupgo® is an investigational topical pan-Janus kinase (JAK) inhibitor for treating moderate to severe CHE. By inhibiting the JAK-STAT signaling pathway, it aims to address the underlying mechanisms of CHE. The cream is currently under development and is not yet approved by health authorities. LEO Pharma holds exclusive rights to develop and commercialize delgocitinib cream worldwide, except in Japan where Japan Tobacco Inc. retains rights.
About LEO Pharma
Founded in 1908 and majority-owned by the LEO Foundation, LEO Pharma is a global leader in dermatology, committed to enhancing the standard of care for skin conditions. Headquartered in Denmark, the company employs approximately 4,200 staff and serves millions of patients globally. In 2023, LEO Pharma reported net sales of DKK 11.4 billion.
Related Topics: