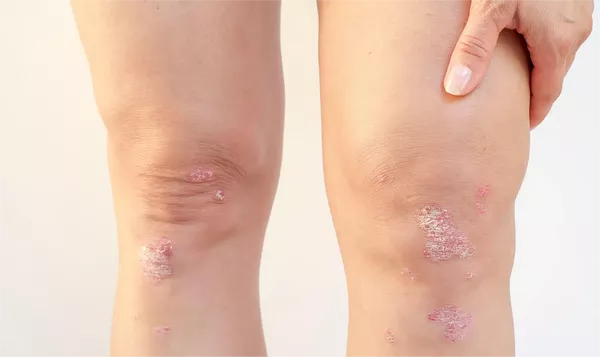
July 23, 2024 – Arcutis Biotherapeutics has officially filed a supplemental new drug application (sNDA) with the U.S. Food and Drug Administration (FDA) for its roflumilast (Zoryve) foam 0.3%, targeting psoriasis treatment for patients aged 12 and older. The proposed treatment is designed to address both scalp and body psoriasis.
Roflumilast foam is a cutting-edge phosphodiesterase-4 (PDE4) inhibitor, administered once daily. The sNDA submission follows positive results from the ARRECTOR phase 3 trial, a pivotal, vehicle-controlled, double-blind study evaluating the safety and efficacy of the foam.
Dr. Melinda Gooderham, a dermatologist at the SKiN Centre for Dermatology in Peterborough, Ontario, shared insights with HCPLive on the significance of the ARRECTOR trial findings and their implications.
“The ARRECTOR study included participants aged 12 and older with psoriasis affecting both the scalp and body. For scalp psoriasis, the severity had to be moderate or severe, while body psoriasis needed to be at least mild, moderate, or severe, with less than 25% body surface area involvement,” Dr. Gooderham detailed.
The study’s primary endpoints were the Scalp-Investigator Global Assessment (S-IGA) and Body-Investigator Global Assessment (B-IGA). According to Dr. Gooderham, the results were promising.
“The trial demonstrated excellent outcomes,” she noted. “Approximately 85% of patients had moderate scalp disease, with 15% presenting severe cases. For body psoriasis, about 25% had mild disease, and two-thirds had moderate disease. Remarkably, two-thirds of patients achieved IGA success for scalp psoriasis, a significant achievement given the challenge of treating this area. For body psoriasis, the IGA success rate was 45%.”
Dr. Gooderham emphasized the impact of roflumilast on pruritus, a common and troublesome symptom of scalp psoriasis. “Pruritus often leads to scratching, which can exacerbate the condition. The trial found a 46.5% B-IGA success rate with roflumilast compared to 20.8% with the vehicle. Furthermore, the Scalp Itch Numeric Rating Scale showed significantly better results within 24 hours for those using roflumilast, suggesting immediate itch relief that could enhance patient adherence to the treatment.”
The submission of the sNDA marks a significant step forward in the fight against psoriasis, offering hope for improved treatment options for patients struggling with this challenging condition.
Related Topics: