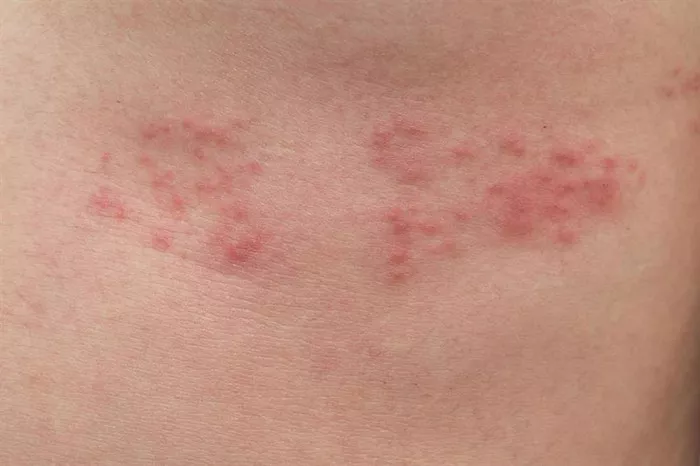
AnaptysBio (ANAB.O) announced on Wednesday that it will cease development of its experimental eczema treatment, ANB032, following disappointing results from a mid-stage clinical trial. The news sent the company’s shares tumbling nearly 40%.
In the Phase 2 trial, which involved 201 participants, ANB032 failed to meet its primary endpoint. The drug did not achieve the expected 75% improvement in eczema symptoms, as measured by the eczema severity index. Additionally, ANB032 did not perform better than a placebo in any of the secondary objectives, including reducing itch severity.
Eczema, also known as atopic dermatitis, is a chronic condition characterized by inflamed, itchy, and red skin. The National Eczema Association estimates that 16.5 million adults in the United States, or approximately 7.3% of the population, are affected by the condition.
While there are various treatments available for eczema, including AbbVie’s Rinvoq, Pfizer’s Cibinqo, Eli Lilly’s Ebglyss, and Sanofi and Regeneron’s Dupixent, the market remains competitive, with several established therapies already in use.
Following the setback, AnaptysBio stated it will shift its focus to other autoimmune treatments in its pipeline, which includes drugs for rheumatoid arthritis and inflammatory bowel disease. CEO Daniel Faga emphasized that the company remains financially strong, projecting a cash balance of around $415 million by the end of 2024. The company also extended its cash runway outlook, now expecting to fund operations through the end of 2027.
Related topics